Abstract:
Background:
Nicotinic acetylcholine receptors (nAChRs) play an important role in cellular physiology and human nicotine dependence, and are closely associated with many human diseases including cancer. For example, previous studies suggest that nAChRs can re-wire gene regulatory networks in lung cancer cell lines. However, the tissue specificity of nAChRs genes and their regulation remain unexplored.
Result:
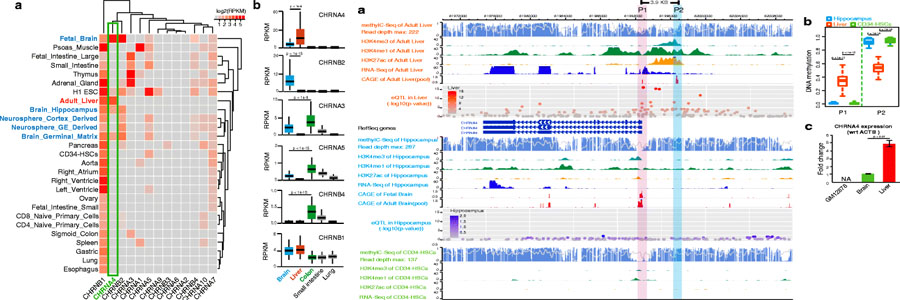
In this study, we integrated data from multiple large genomic consortiums, including ENCODE, Roadmap Epigenomics, GTEx, and FANTOM, to define the transcriptomic and epigenomic landscape of all nicotinic receptor genes across many different human tissues and cell types. We found that many important nAChRs, including CHRNA3, CHRNA4, CHRNA5, and CHRNB4, exhibited strong non-neuronal tissue-specific expression patterns. CHRNA3, CHRNA5, and CHRNB4 were highly expressed in human colon and small intestine, and CHRNA4 was highly expressed in human liver. By comparing the epigenetic marks of CHRNA4 in human liver and hippocampus, we identified a novel liver-specific transcription start site (TSS) of CHRNA4. We further demonstrated that CHRNA4 was specifically transcribed in hepatocytes but not transcribed in hepatic sinusoids and stellate cells, and that transcription factors HNF4A and RXRA were likely upstream regulators of CHRNA4. Our findings suggest that CHRNA4 has distinct transcriptional regulatory mechanisms in human liver and brain, and that this tissue-specific expression pattern is evolutionarily conserved in mouse. Finally, we found that liver-specific CHRNA4 transcription was highly correlated with genes involved in the nicotine metabolism, including CYP2A6, UGT2B7, and FMO3. These genes were significantly down-regulated in liver cancer patients, whereas CHRNA4 is also significantly down-regulated in cancer-matched normal livers.
Conclusions:
Our results suggest important non-neuronally expressed nicotinic acetylcholine receptors in the human body. These non-neuronal expression patterns are highly tissue-specific, and are epigenetically conserved during evolution in the context of non-conserved DNA sequence.